5/1/2017
Contraception for Women with Diabetes
Registered users can also download a PDF or listen to a podcast of this Pearl.
Log in now, or create a free account to access bonus Pearls features.
The prevalence of diabetes mellitus is increasing worldwide, including among women of childbearing age, with an additional 150 million adult cases projected by 2030. According to the U.S. Medical Eligibility Criteria (MEC) for Contraceptive Use, updated in 2024, non-hormonal birth control methods such as barrier methods and the copper IUD (Cu-IUD) are classified as having no restriction for patients with diabetes-related diseases. Additionally, methods like the levonorgestrel-IUD, implant, and progestin-only pill are generally considered to have advantages that outweigh any theoretical or proven risks. Diabetes is an independent risk factor for both micro- and macrovascular disease, so contraceptives that do not exacerbate these risks are preferred. At the same time, the risk of unplanned pregnancy, particularly in the setting of poor glycemic control, must be carefully weighed against the potential risks of the contraceptive use.
There is no indication for diabetes screening prior to the initiation of any form of contraception. Concern has been raised about the impact of hormonal contraception on glucose metabolism; however, no short or long-term effects have been proven. After detailed review of the diabetic patient’s medical history, hormonal contraceptive methods can be considered. Combined hormonal contraceptives (CHC) do not increase the risk of developing diabetes, even in patients with a history of gestational diabetes. CHC are considered safe and effective for women with diabetes that is not complicated by vascular disease (MEC Category 2). CHC can be prescribed to women with insulin-dependent diabetes unless they have severe microvascular disease such as retinopathy, nephropathy, and neuropathy, or the duration of disease is greater than 20 years (MEC Category 3 or 4 depending on severity). As estrogen-containing contraceptives are classified as MEC Category 3 or 4 in women with multiple risk factors (e.g., diabetes, smoking, hypertension) for atherosclerotic cardiovascular disease, women with diabetes should have their blood pressure evaluated prior to and monitored periodically after initiating CHC.
Progesterone-only contraceptive methods, including pills, injections, implants, and the levonorgestrel IUD (LNG-IUD), are generally considered safe for women with diabetes, including those with microvascular disease or diabetes of more than 20 years’ duration. These methods have minimal impact on glucose metabolism, lipids, and thrombotic markers. An important exception is depot medroxyprogesterone acetate (DMPA). The theoretical or proven risks of DMPA in diabetics with vascular disease or duration of > 20 years are thought to outweigh the benefits, due to the increased risk of atherosclerosis. DMPA use under these circumstances is classified as MEC category 3 and is generally not recommended.
Sterilization is an option for diabetic patients who have completed childbearing. Surgical complications can be reduced by optimizing glycemic control at the time of the procedure.
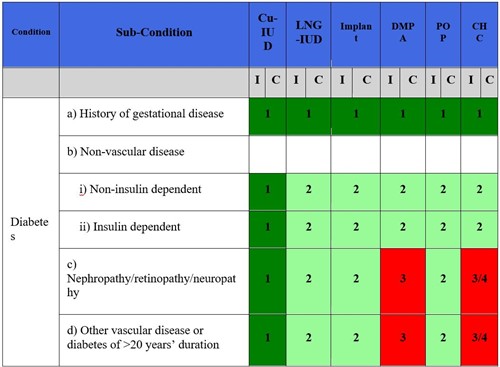
1 = No restriction for the use of the contraceptive method.
2 = Advantages of using the method generally outweigh the theoretical or proven risks.
3 = Theoretical or proven risks usually outweigh the advantages of the method.
4 = Unacceptable health risk if the contraceptive method is used.
Further Reading:
Nguyen AT, Curtis KM, Tepper NK, Kortsmit K, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2024. MMWR Recomm Rep. 2024 Aug 8;73(4):1-126. doi: 10.15585/mmwr.rr7304a1. PMID: 39106314; PMCID: PMC11315372.
Curtis KM, Nguyen AT, Tepper et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2024. . MMWR Recomm Rep. 2024 Aug 8;73(3):1-77. doi: 10.15585/mmwr.rr7303a1. PMID: 39106301; PMCID: PMC11340200.
Summary Chart of U.S. Medical Eligibility Criteria for Contraceptive Use, 2024 available at: https://www.cdc.gov/mmwr/volumes/73/rr/pdfs/rr7304a1-H.pdf
ACOG Practice Bulletin No. 206: Use of Hormonal Contraception in Women With Coexisting Medical Conditions. Obstet Gynecol. 2019 Feb;133(2):e128-e150. doi: 10.1097/AOG.0000000000003072. Erratum in: Obstet Gynecol. 2019 Jun;133(6):1288. doi: 10.1097/AOG.0000000000003332. PMID: 30681544.
Initial Approval: January 2017; Reaffirmed July 2018; Reaffirmed and title revised January 2020; Revised September 2021. Revised May 2023. Revised January 2025.
Initial Approval: January 2017; Reaffirmed July 2018; Reaffirmed and title revised January 2020; Revised September 2021. Revised May 2023. Revised January 2025.
********** Notice Regarding Use ************
The Society for Academic Specialists in General Obstetrics and Gynecology, Inc. (“SASGOG”) is committed to accuracy and will review and validate all Pearls on an ongoing basis to reflect current practice.
This document is designed to aid practitioners in providing appropriate obstetric and gynecologic care. Recommendations are derived from major society guidelines and high-quality evidence when available, supplemented by the opinion of the author and editorial board when necessary. It should not be construed as dictating an exclusive course of treatment or procedure to be followed.
Variations in practice may be warranted when, in the reasonable judgment of the treating clinician, such course of action is indicated by the condition of the patient, limitations of available resources, or advances in knowledge or technology. SASGOG reviews the articles regularly; however, its publications may not reflect the most recent evidence. While we make every effort to present accurate and reliable information, this publication is provided “as is” without any warranty of accuracy, reliability, or otherwise, either express or implied. SASGOG does not guarantee, warrant, or endorse the products or services of any firm, organization, or person. Neither SASGOG nor its respective officers, directors, members, employees, or agents will be liable for any loss, damage, or claim with respect to any liabilities, including direct, special, indirect, or consequential damages, incurred in connection with this publication or reliance on the information presented.
Copyright 2025 The Society for Academic Specialists in General Obstetrics and Gynecology, Inc. All rights reserved. No re-print, duplication or posting allowed without prior written consent.
Back to Search Results
