3/1/2017
Contraception for Patients with Migraines
Registered users can also download a PDF or listen to a podcast of this Pearl.
Log in now, or create a free account to access bonus Pearls features.
Migraine headache is a common condition that affects up to 24% of females aged 18-39. A migraine is a headache lasting 4 to 72 hours and must have nausea, vomiting, or photophobia, as well as at least two of the following: unilateral location, pulsating quality, moderate to severe pain, and aggravation by routine physical activity.
The two categories of migraines include those with aura (classical) and those without aura (simple). Aura is a completely reversible focal neurological sensory event that can precede or accompany the headache, come on gradually, and last no longer than one hour. Twenty-five percent of patients with migraines experience aura. The most common aura is visual and typically includes a bright spot or an area of visual loss. The aura then expands to involve a quadrant or hemifield of vision, often with “zigzagging” lines. Other types of aura include somatosensory phenomena, such as burning or paresthesias that migrates across one side of face or down the arm. Less frequently, auras can include motor or verbal symptoms.
There is an increased risk for cerebral thromboembolism (CTE) in individuals who use combined hormonal contraception (CHC), have migraines, have hypertension, or use tobacco. These factors have a multiplicative effect on risk for CTE. Individuals who experience migraines with aura and use CHC increase their risk of CTE two-fold, although the absolute risk is low and less than during pregnancy. Individuals with a history of migraine w
Risk for CTE per 100,000 women/years:

Migraines without aura may worsen, improve, or remain stable in individuals who take CHCs. While on CHCs, migraines typically occur during the hormone-free interval due to estrogen withdrawal. Using continuous dosing with a shortened or absent hormone free interval typically alleviates these headaches. Menstrual migraines are considered a subtype of migraines without aura and are similar to the migraines triggered by a hormone free interval during CHC use. Menstrual migraines will often resolve with similar interventions. Individuals who start a new hormonal contraceptive should notify their provider if their headaches worsen.
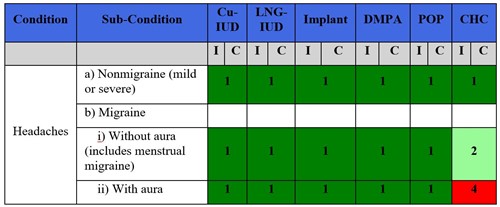
From the US Medical eligibility Criteria
1 = no restriction for the use of the contraceptive method;
2 = advantages of using the method generally outweigh the theoretical or proven risks;
3 = theoretical or proven risks usually outweigh the advantages of the method;
4 = unacceptable health risk if the contraceptive method is used.
Further Reading:
Curtis KM, Tepper NK, Jatlaoui TC, et. al, U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016 Jul 29;65(3):1-103. doi: 10.15585/mmwr.rr6503a1. PMID: 27467196.
Tepper NK, Whiteman MK, Zapata LB, Marchbanks PA, Curtis KM. Safety of hormonal contraceptives among women with migraine: A systematic review. Contraception. 2016 Dec;94(6):630-640. doi: 10.1016/j.contraception.2016.04.016. Epub 2016 May 3. PMID: 27153744.
American College of Obstetricians and Gynecologists, ACOG Practice Bulletin No. 206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019 Feb;133(2):e128-e149.
Initial Approval: January 2017; Revised July 2018; Revised January 2020; Revised September 2021
Originally Titled “Contraception for Women with Migraines”. Retitled May 2023.
********** Notice Regarding Use ************
The Society for Academic Specialists in General Obstetrics and Gynecology, Inc. (“SASGOG”) is committed to accuracy and will review and validate all Pearls on an ongoing basis to reflect current practice.
This document is designed to aid practitioners in providing appropriate obstetric and gynecologic care. Recommendations are derived from major society guidelines and high-quality evidence when available, supplemented by the opinion of the author and editorial board when necessary. It should not be construed as dictating an exclusive course of treatment or procedure to be followed.
Variations in practice may be warranted when, in the reasonable judgment of the treating clinician, such course of action is indicated by the condition of the patient, limitations of available resources, or advances in knowledge or technology. SASGOG reviews the articles regularly; however, its publications may not reflect the most recent evidence. While we make every effort to present accurate and reliable information, this publication is provided “as is” without any warranty of accuracy, reliability, or otherwise, either express or implied. SASGOG does not guarantee, warrant, or endorse the products or services of any firm, organization, or person. Neither SASGOG nor its respective officers, directors, members, employees, or agents will be liable for any loss, damage, or claim with respect to any liabilities, including direct, special, indirect, or consequential damages, incurred in connection with this publication or reliance on the information presented.
Copyright 2023 The Society for Academic Specialists in General Obstetrics and Gynecology, Inc. All rights reserved. No re-print, duplication or posting allowed without prior written consent.
Back to Search Results